Plasma Separation
Wolfgang Steurer
This process employs a thermal plasma for the separation and production of oxygen and metals. It is a continuous process that requires no consumables and relies entirely on space resources. The almost complete absence of waste renders it relatively clean. It can be turned on or off without any undesirable side effects or residues. The prime disadvantage is its high power consumption.
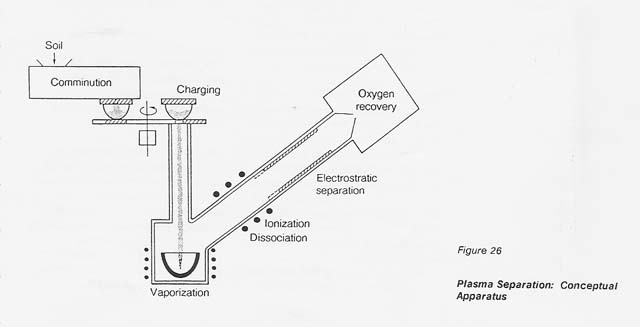
In the basic concept, the process consists of the following steps: Granulated raw material, such as lunar regolith, is vaporized, dissociated, and finally brought to a temperature where a thermal plasma is obtained. The plasma permits electromagnetic manipulation and separation of the ionized species according to their positive or negative charges.
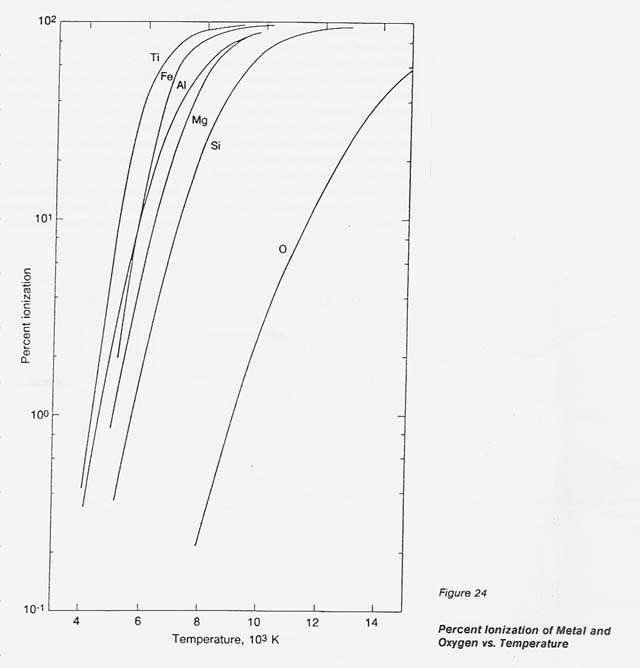
In the process discussed here, a unique concept is introduced and designated" selective ionization.'! Metals exhibit a low ionization potential, below 9 eV, while the lowest ionization potential of gases is 13.6 eV (oxygen). In a thermal plasma, where the degree of ionization is related to temperature, this gap in the ionization potential defines a temperature range, between 8 000 and 10 000 K, in which metals approach 100- percent ionization while the atomic oxygen remains essentially neutral (ionized 02 less than 1 percent). Under these conditions, only metals respond to electromagnetic forces and, consequently, can be separated from the neutral oxygen.
To substantiate this effect, theoretical data were generated for the metals of interest and for oxygen by programming the well- accepted Saha equation. The data on the number densities of the positive ions, neutral species, and electrons at temperatures from 4 000 to 14 000 K were translated into percent ionization. The results, plotted in figure 24, clearly show the wide gap in the degree of ionization for the metals concerned and oxygen between 8 000 and 10 000 K.

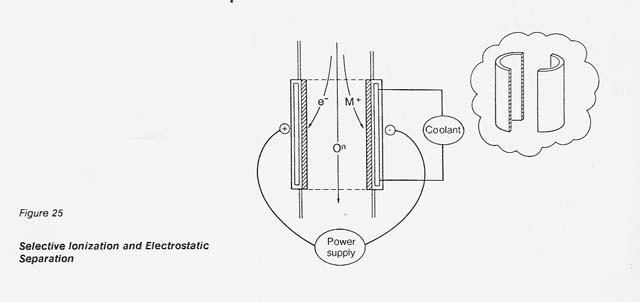
In the proposed process, the dissociated gas is heated to 9 000 K by inductive coupling and the selectively ionized plasma is passed through an electrostatic field for separation. As shown in figure 25, the positive metal ions are diverted toward the cathode half-shell. The neutral oxygen continues to flow downstream and is recovered at the end of the column in an appropriate collection system.
A conceptual processing facility is shown in figure 26. The ions of each individual metal follow a specific trajectory and deposit, therefore, at distinct distances in the electrostatic system. This fact implies the possibility of recovering individual metals rather than a metal mixture.
While the process is basically continuous, it requires periodic removal of the metal deposits from the cathode. Since there is essentially no waste, the combined process yield of metals and oxygen approaches 100 percent. Conservative yield factors (fraction of throughput) are as follows:

Total energy requirements are approximately 13 300 kWhr per tonne of all products. Of this, 4500 kWhr/t can be satisfied by direct solar heating/vaporization /dissociation. The remaining 8800 kWhr/t has to be supplied in the form of electric power.
This figure has to be increased by a factor of 2.2 for power conditioning and losses, resulting in an actual power consumption of 19 360 kWhr/t. A yearly combined production of 500 tonnes of metals and oxygen, equivalent to 125 kg per hour, calls for an electric power generation capacity of 2400 kW (installed).
This assessment is based on the use of inductive heating for plasma generation. There may be other alternatives, such as laser heating, which may simplify the problem of plasma containment. However, the effect of such alternate concepts on power requirements is not expected to be substantial.