Processes for Metal Extraction
David F. Bowersox
The cost of transportation from Earth to the Moon is so high that proposals for industrial efforts on the Moon are often limited to native (lunar) materials. This restriction, fortunately, can be greatly eased if recyclable elements are shipped from Earth and reused. The initial, nearly prohibitive costs are alleviated by the repeated operation. The expenses of the reagents, like those of the shelter and special equipment, are spread over a relatively long program. This report describes the processing of plutonium at Los Alamos National Laboratory (LANL), an operation illustrating concepts that may be applicable to the processing of lunar materials. The toxic nature of plutonium requires a highly closed system, just as the expense of transporting reagents to the Moon requires a highly closed system for processing lunar surface materials.
To illustrate the benefit of using a closed pyrometallurgical process on the Moon, let us take the reduction of ilmenite as an example. Ilmenite ore can be isolated and beneficiated, and usable quantities of oxygen, iron, and titanium can be extracted from the ore. The first step might be a hydrogen reduction step, in which hydrogen is reacted with the ilmenite ore to produce water and a slag of iron mixed with titanium dioxide. By following this step with electrolysis of the water, we can recover the hydrogen and produce oxygen for life support and propulsion.
Let us now consider expanding the ilmenite process by adding a step in which the iron-titania slag is treated so as to obtain metallic iron and titanium dioxide or, even better, metallic iron and metallic titanium. Two additional processes are suggested-a carbonyl process for separating the metallic iron from the slag or from titanium and a pyrochemical process to produce titanium metal.
The carbonyl process would separate iron (or nickel or other metals) from titania (TiO2) or titanium metal by the reaction
![]()
The compound can then be decomposed and the carbon monoxide recycled. This well- known method is compact and requires very little power.
The pyrochemical reduction of TiO2 to
metallic titanium may be carried out in a manner analogous to the process used
to extract plutonium from scrap residues. Although the process is not directly
applicable to nonterrestrial industrialization in its detailed
steps, the success of this method indicates that it should be excellent for
space programs if it can be applied to the extraction of other metals.
First, because of the solubilities and densities of the phases, the system is compact. Pyroprocessing requires approximately one-tenth the volume of aqueous processing. Operations could be remote and automated. Processes are either batch or semicontinuous, depending on the desired throughput. Reagents are generally recyclable, and residues, when produced, are in compact, dense form.
The major steps for plutonium processing are outlined in figure 27. The roasting step (1) would not be necessary in any nonterrestrial application; the starting material would already be in oxide form. The oxide, calcium metal, and excess CaCI2 are reacted (step 2) in a magnesium oxide crucible where the plutonium is reduced to the metal by the reaction
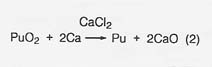
The calcium oxide dissolves in the calcium chloride and the metallic plutonium settles as a button, which is mechanically separated from the salt. In a nonterrestrial application, titanium and iron could form a metal button and then be separated by vacuum distillation or the carbonyl process. And the reactant metal could be calcium or perhaps aluminum. We at LANL are developing a method for recovering the calcium chloride for recycling.
If, in the plutonium process, the americium concentration is greater than 1000 ppm, it is lowered by equilibration at 800°C with sodium chloride/potassium chloride eutectic salt containing magnesium chloride as an oxidizing agent (step 3). The reactions are
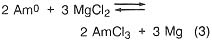
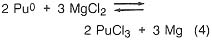
![]()
Under the conditions of the plutonium process, the salt contains most of the americium and 4 percent of the plutonium, while the plutonium, magnesium, and about 100 ppm americium are in molten form. The salt can be treated with calcium to extract the americium and plutonium and subsequently be reused. Although this is an important part of the plutonium process, it would not be necessary in a titanium recovery process. In both cases, however, because there is excess calcium and magnesium in the metal button, heating above the melting point is necessary to remove these volatiles. The product of this step is a solid metal, typically in the form of a solid metal cylinder formed by chill casting.
In the fourth step, purification of the metal, the cylinder of plutonium is placed in the anode cup of a magnesium oxide crucible, a sodium chloride/potassium chloride eutectic added, and electrolysis conducted at 750°C. The impure plutonium is ionized by giving up electrons at the anode. Then the ions migrate to the cathode to get electrons and deposit as pure plutonium. The reactions are
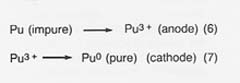
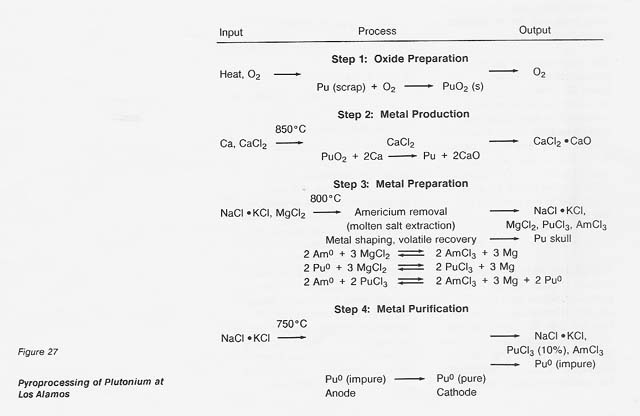
Approximately 10 percent of the plutonium is in the salt phase and 10 percent remains in the anode as a solid when the electrorefining is completed. The cathode metal is 99.99-percent pure plutonium. The salt can be reused or treated to remove the plutonium trichloride, and the anode metal can be oxidized to remove impurities and then reduced in the metal production step.
This process has been developed over a period of years and is
used successfully at Los Alamos National Laboratory. Changes are being made
to optimize the process. However, the concept, with its compactness and its
recycling of reagents, seems particularly transferable to nonterrestrial processing.
The process requires no water, and the availability of high vacuum and heat
sources (such as a solar furnace) should be advantageous in developing a successful
process off Earth. In the space context, the availability of a reactant metal
may be a problem. If aluminum could be scavenged from space vehicles, or lunar
calcium be used and recycled, the economics would be enhanced.