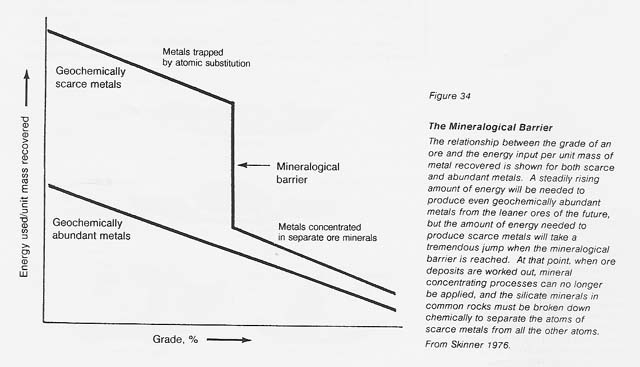
The geochemically scarce metals are those which do not normally form separate minerals but are present as substitutional atoms in common rock; for instance, lead or zinc may occur at ppm levels in orthoclase (KAISi308). To release lead from the silicate structure, the entire mineral has to be chemically broken down, an extremely complex and energy-intensive process. "The mineralogical barrier" described by Skinner (see fig. 34) refers to the point at which the easily processed minerals, such as sulfides, are so rare that mineral beneficiation techniques cannot be applied economically. At this level, the increased energy required to extract trace metals from silicates must be expended. Although the diagram is conceptual, the energy jump for the mineralogical barrier is 2 to 3 orders of magnitude higher than the energy required for the processing of typical ore minerals on Earth.
On Earth, mainly by means of free water, nature has concentrated the rarer metals in are deposits. Such deposits are unknown on the dry Moon. On the Moon, metallic iron can be concentrated from soils, and iron and titanium oxides (ilmenite) and iron sulfides (troilite) can be concentrated from soils or disaggregated rocks. Essentially all other known lunar ores are above the mineralogical barrier and will require considerable energy for their extraction. Examples include silicon and aluminum from anorthite and iron or magnesium from pyroxene.

Extraction Technology
Extraction of various metals from mineral ores has developed into three subdisciplines: pyrometallurgy, electrometallurgy, and hydrometallurgy. In each subdiscipline, a different mechanism provides the driving force to reduce the combined metal to its elemental form.
In pyrometallurgy the force that drives the chemical reduction of a metal oxide is high temperature. For many of the less stable metal oxides, carbon reduction at elevated temperatures is possible. This technology has been used successfully for iron smelting. (See figure 35.) For the more stable metal oxides, such as TiO2 and A1203, carbon reduction proceeds spontaneously (that is, the free energy becomes negative) only above 1630�C and 2000�C, respectively. The disadvantages of high temperature extraction include the large amount of energy required for heating, the difficulty in finding suitable container materials, and the problem of reoxidation of the molten metal.
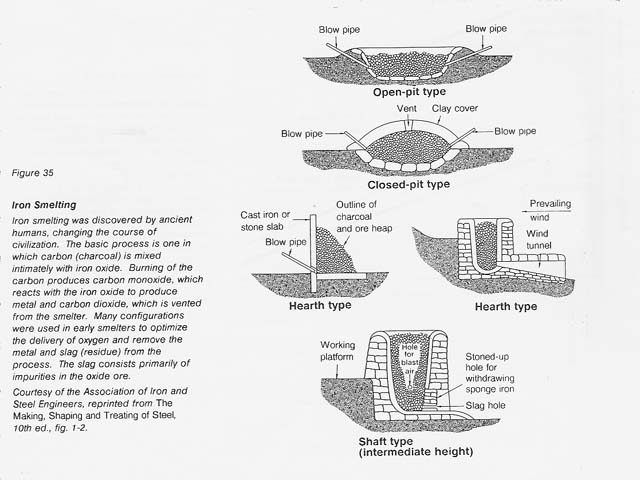
the large amount of energy required for heating, the difficulty in finding suitable container materials, and the problem of reoxidation of the molten metal.
In electrometallurgy an electrical rather than a chemical driving force is used to reduce the metal oxide. An example of a well- developed electrometallurgical technology is the extraction of aluminum from AI203 by electrolysis in molten fluoride salts.
Hydrometallurgy exploits the fact that certain minerals are soluble in aqueous solutions such as sulfuric acid. Once dissolved, the metal ions can be recovered by low- temperature electrolysis, precipitation, chemical reduction, ion exchange, or solvent extraction. (See figure 36.)

The scarcity of water and the abundance of solar energy available at nonterrestrial sites suggest that pyrometallurgy or electrometallurgy will be favoredas processing technologies there. Processes that conserve volatile substances by recycling will be essential to minimize the transport of makeup chemicals, as will be processes that limit the consumption of special materials, such as fluxes or anodic substances, which would have to be transported to space.
This discussion of geochemical availability and extractive metallurgy implies that extraction of minor elements in space is questionable unless specific natural concentrations are discovered or energy becomes very inexpensive. The relative costs of scarce and abundant metals will become even more disparate in the future on Earth as well as in space. It may be more cost-effective to substitute the lesser properties of an abundant metal like iron or aluminum than to attempt to extract a geochemically scarce metal. This line of thought suggests that effort be directed to extracting iron on the Moon rather than to recovering scarcer metals (such as those from the platinum group) from asteroids. When exploration and timing difficulties are added to the energy consumption considerations, the case for going after the rarer precious metals becomes yet weaker.