Reduction of Igneous Rock With Carbon and Silicon Carbide
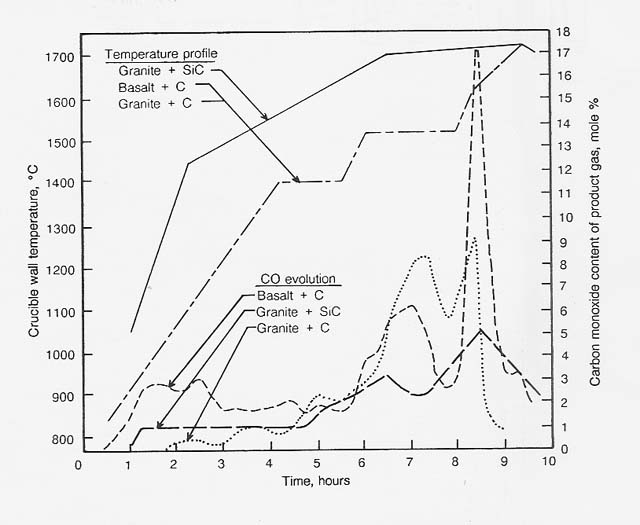
A series of reactions of basalt and granite with carbon and silicon carbide were carried out to determine the temperature profile for the reduction reactions that may occur during the reduction of igneous rock with methane. The results of three of these runs are illustrated in figure 9.

Figure 9
Reduction of Basalt and Granite With Carbon and Silicon Carbide
In the reaction of basalt (50 g) with carbon (5 g), the initial
evolution of carbon monoxide resulted from the reduction of iron oxide. The
basalt contained 11.86 percent iron oxide (as Fe203);
the reduction of this oxide, if present as Fe203,
would require 1.34 9 of carbon. The carbon monoxide that evolved during the
first 2.5 hours represented 1.0 9 of the carbon. Other reducible materials present
in the basalt were titanium dioxide (2.47%) and sodium oxide (3.73%.). These
oxides would consume 0.43 9 of carbon. Consequently, only 35 percent of the
carbon could have been oxidized by materials other than silicon dioxide. The
recovery in the form of carbon monoxide of 89.1 percent of the carbon with which
the reactor was charged indicates that a considerable portion of the silicon
dioxide present in the basalt was reduced at temperatures as low as 1550°C.
Three solid products were obtained: slag and metal remained in the zirconium dioxide crucible and subl!mate was found at the top of the bell jar. The slag was composed mainly of aluminum oxide. The composition of the metal was 82 percent iron, 13 percent silicon, and minor amounts of titanium, vanadium, nickel, and copper. Of the sublimate, 61 percent was sodium, which is highly volatile.
In the reaction of granite (50 g) with carbon (5 g), much less carbon monoxide was produced at low temperature. This result is due to the lower percentages of reducible oxides in the granite; that is, iron oxide (2.05% as Fe203), sodium oxide (3.10%), and potassium oxide (4.90%). Complete reduction of these oxides would consume 0.85 g (17%) of the carbon introduced. A total of 73 percent of the carbon introduced was recovered as carbon monoxide; therefore, we conclude that silicon dioxide reduction accounts for most of the carbon monoxide evolved at 1550°C and higher temperatures.
We believe that most of the rest of the carbon introduced reacted with silicon to form silicon carbide. The slag had nonmagnetic pieces of metal dispersed throughout and contained 2.3 percent carbon; that is, 20 percent of the carbon introduced.
In the reaction of granite (37.5 g) with silic6n carbide (12.5 g), almost no reaction occurred below 11 00 ° C; about 7 percent of the silicon carbide was reacted between 1100 and 1500°C. As the temperature was increased from 1500 to 1740 ° C, the reaction rate gradually increased and then rapidly decreased when most of the carbon was consumed. About 83 percent of the carbon in the silicon carbide was recovered as carbon oxides. The dark, metallic looking slag contained an additional 10 percent of the carbon introduced as silicon carbide.
Analysis of the metal recovered from the melt gave 59 percent iron, 28 percent silicon, and minor amounts of titanium, vanadium, nickel, and copper. The slag was composed mainly of aluminum oxide and silicon dioxide.
These results indicate that, if silicon carbide is formed by reaction of granite and carbon, excess granite will react with the carbide to produce silicon and carbon monoxide. The rate of the granite- siticon carbide reaction at 1740°C is comparable to that of the granite- carbon reaction at 1625 ° C.