Magnetic Concentration
Unlike Earth soils, all lunar soils contain naturally occurring, particulate iron/nickel metal (FeNi), which is believed to derive from meteorite impacts. Lunar soil metal is likely to be an accessible and useful resource. Goldstein and his associates (1972, 1973) reported soil metal contents of 0.15 percent by weight in the size range 74 pm to 1.0 mm of three Apollo 16 soils and 0.05 percent by weight in a comparable size range of two Apollo 14 soils. If these occurrences are typical of the lunar highlands and maria, respectively, then there are at least 7 billion metric tons of accessible FeNi metal in the top 10 cm of soil over the entire lunar surface. And there may be substantially higher concentrations of surface metal in regions where iron meteorites have struck the Moon.
In 1981, I proposed a magnetic beneficiation method for concentrating lunar soil metal, using off-the-shelf permanent magnet separators and autogenous grinders. Projected yield was 552 metric tons per year of 99- percent pure FeNi powder. The specific energy required to extract the FeNi metal magnetically was 0.4 kWh/kg, an order of magnitude less than that required to smelt iron from typical ores. A major advantage of the concentrated metal powder product is that it may be formable directly by flexible, low-power powder metallurgical techniques to make a variety of tools, machine parts, plates, struts, wires, electrical contacts, and magnets. Near-theoretical density for these parts may be achievable by powder pressing in the high lunar vacuum. Furthermore, the product can be toughened to steel specifications by adding the right proportions of lunar oxides or titanium to,the metal powder before pressing.
An all-magnetic method for beneficiating soil FeNi may present problems because of the large volume of iron-bearing agglutinates that have ferromagnetic properties. But Goldstein did achieve concentration of soil metal grains in the laboratory, using a very low magnetic field gradient on the Frantz Isodynamic Magnetic Separator, and a comparable technique might be adaptable to industrial operations on the Moon.
I suggest that metal particles first be separated from ilmenite and other soil components magnetically and then, because the ferromagnetic agglutinates may have separated with the metal particles, electrostatic separation could be used to eliminate the agglutinates from the desired metal fraction. Comparable combinations of techniques may be appropriate for extracting other soil components, like anorthite, chromite, and phosphates.
Lunar Soil Sizing
What volatiles are known to exist on the Moon tend to be concentrated in the fine soil fractions. For example, Gibson et al. (1987) showed that hydrogen implanted by the solar wind increases tenfold as particle size decreases-':'from 12 ppm in the 90- to 150-micrometer fraction to 127 ppm in the less-than-20- micrometer fraction of five representative regolith samples. Overall hydrogen content is about 40 ppm. At 75-percent recovery from the top meter of soil over the entire lunar surface, that is enough hydrogen to make a water lake 10 meters deep and 44 kilometers in diameter. The helium content of the soil is about the same as the hydrogen content. Furthermore, 0.04 percent of the lunar helium is the isotope 3He, which is much rarer on Earth and which is a potentially important fusion energy fuel (Wittenberg, Santarius, and Kulcinski 1986). Helium-3 may be the only lunar product that can be returned to the Earth at a substantial profit. Accordingly, lunar soil sizing techniques will be vital to extracting rare and precious lunar volatiles. In addition, sized soil input is required to optimize mineral yield by electrostatic and magnetic separation methods.
Dry sizing techniques that may be appropriate to the lunar environment include electrical sizing, screening, and gas elutriation.
Electrical Sizing
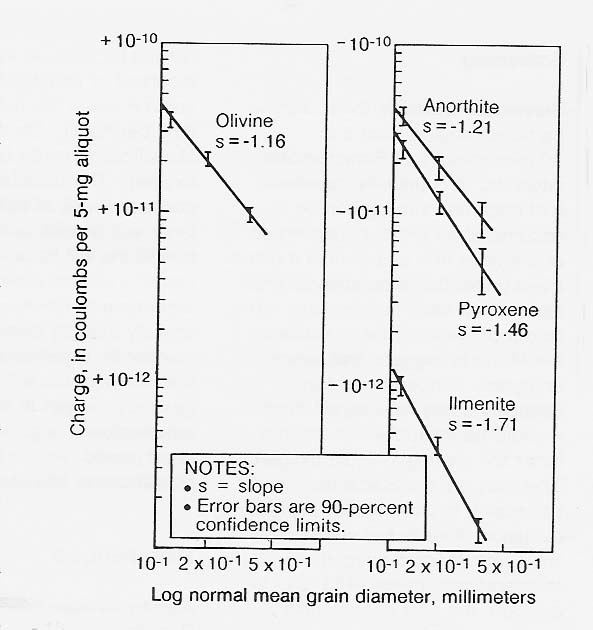
In 1984, I measured the trend of increasing charge-to-mass ratio with decreasing grain size in terrestrial analogs of common lunar regolith minerals and subsequently demonstrated electrostatic sizing of terrestrial ilmenite over the particle size range of 500 down to 90 micrometers (figs. 5 and 6). The previous year, Peter Castle, at the University of Ontario, demonstrated ac electrical sizing of conducting spheres in- a comparable size range. In both cases, air turbulence limited the smallest separable size to 90 micrometers. Accordingly, electrical sizing in a vacuum is indicated for grading of fines smaller than 90 micrometers.

Figure 5
Contact Charge Acquired on Aluminum by Terrestrial Olivine, Anorthite, Pyroxene, and Ilmenite as a Function of Grain Size
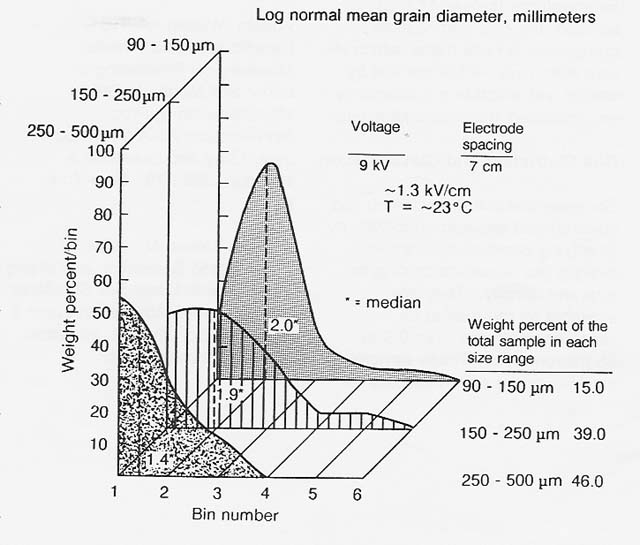
Figure 6
Electrostatic Sizing of Comminuted Ilmenite