Lunar Beneficiation
William N. Agosto
With the exception of igneous differentiation and possible fumarolic activity (volcanic exhalations),
the Moon lacks all the major ore-forming processes that operate on Earth-aqueous concentration of crustal minerals,
surface weathering of rocks, advanced fractionation of igneous rocks, and plate tectonic recycling of the crust. For
that reason, natural concentrations of industrially valuable minerals (ore bodies) are far less likely to be found
on the Moon than on the Earth (see James Carter's paper, earlier in this volume). But that is all the more reason
for devising beneficiation processes to concentrate and extract the useful mineral components in lunar rocks and soils.
Another important consideration is that nearly complete reagent recycling will be required for most of the processes
proposed for producing oxygen, metals, and ceramics on the Moon. Reagent recovery will be greatly simplified by using
simple input ore minerals. Examples of such minerals are ilmenite, the most abundant lunar oxide and a source of oxygen,
iron, and titanium; anorthite, the chief source of lunar aluminum; and metallic iron/nickel fragments that occur in lunar
soil. In addition, there may be significant amounts of chromite, sulfides, and phosphates in terranes that are rich
in chromium and KREEP (potassium, rare earth elements, and phosphorus). As an example of a useful mineral that can be beneficiated, McKay and Williams (1979) have estimated
ilmenite abundance by microscopic count to be 15 and 20 percent by volume in Apollo 11 and 17 basalts and 2 and 5
percent by volume in Apollo 11 and 17 soils. Reduction of lunar ilmeQite with hydrogen imported from Earth appears to
be one of the more practical schemes for obtaining lunar oxygen. While the reported concentrations are significant, a
more highly concentrated ilmenite extract would improve the efficiency of the reduction process. Electrostatic Concentration

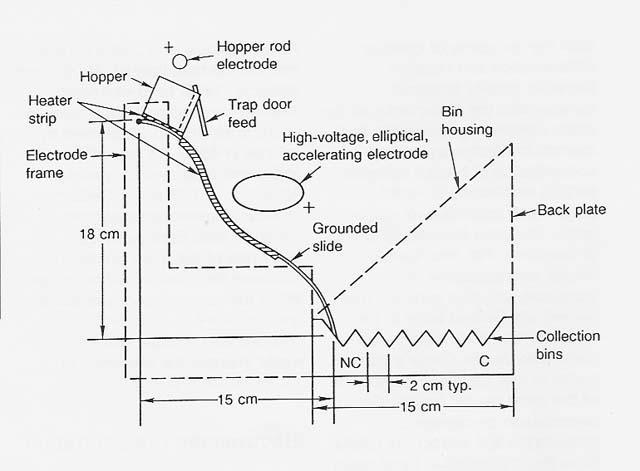
Figure 1
Mineral Electrostatic Separator, Slide Configuration
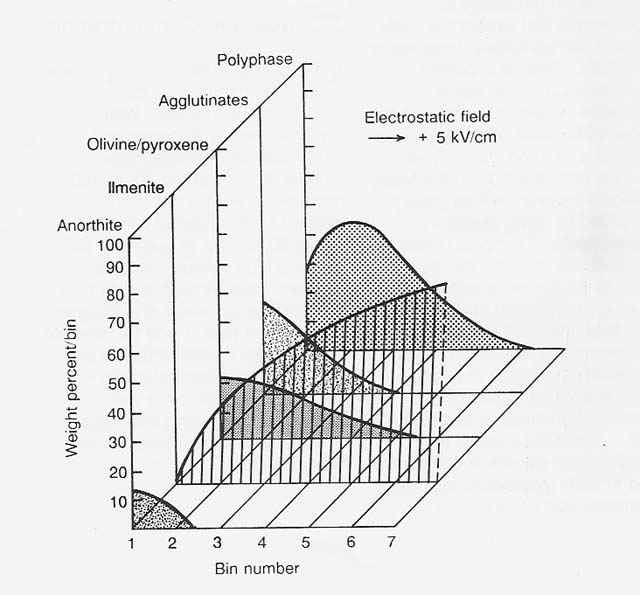
Figure 2
Electrostatic Separation in Nitrogen of Apollo 11 Soil 10084,853, 90.150 11m Fraction