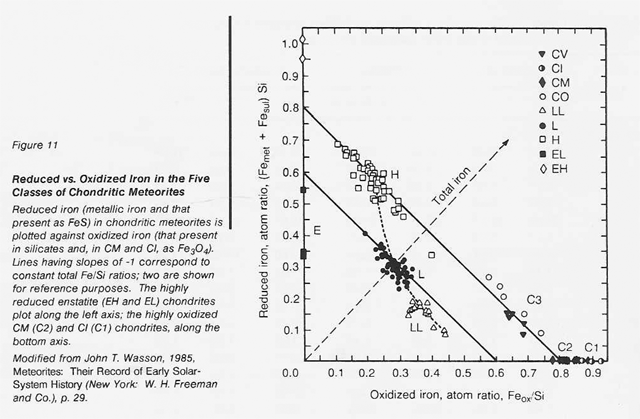

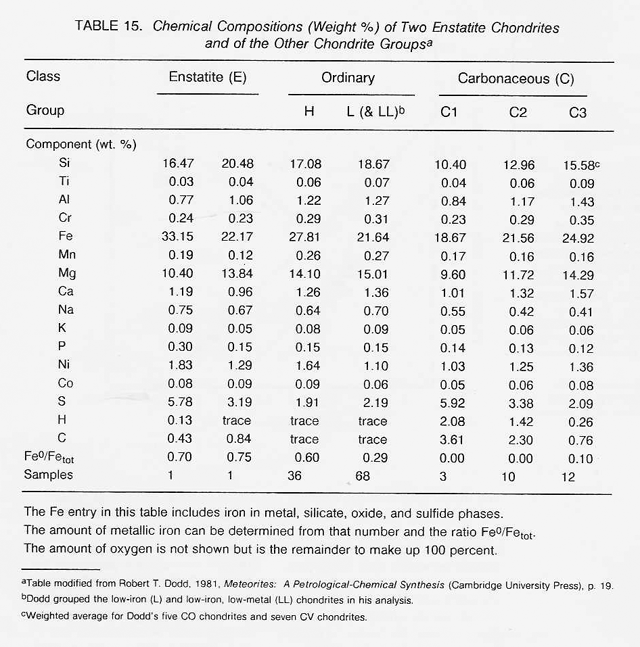
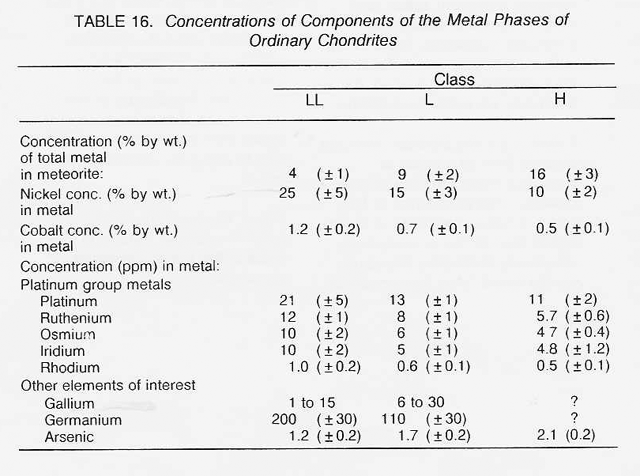
The most highly oxidized ordinary chondrites (LL) and the most volatile-poor and unoxidized of the carbonaceous chondrites (C3) contain by weight only 16 percent free metal. But, because the less abundant components of the metal (nickel, cobalt, and the platinum group metals) are harder to oxidize than iron, they have been concentrated in the metal grains. Thus. the metal grains in these more oxidized chondrites contain far greater concentrations of these metals than do the metal grains in, say, the E chondrites. Nickel ranges from about 6 percent of the metal in E chondrites to 60 percent in the C3 chondrites, and cobalt and the platinum group follow suit. In each class of chondrites, the concentration of the more valuable elements in the metal phase is highest in the smallest grains. Table 16 shows the concentrations of a number of elements in the magnetically separable (metallic) component of chondritic meteorites. It can be seen that the fourfold overall depletion of the metallic elements in the LL chondrites relative to H chondrites is accompanied by only a twofold depletion in their platinum group content. It is thus not terribly important which class of ordinary chondrites is exploited for these elements. Magnetic extraction of iron-rich phases (magnetite, FeS, etc.) might be applied to a C1 chondrite, which is lacking in free metal, since C1 chondrites contain by weight up to 11 percent magnetite and about 2 percent nickel-rich sulfides. However, it has not yet been shown that such a separation process is practical.
Next