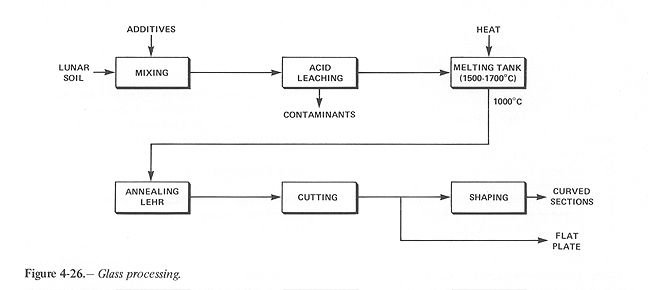
GLASS PROCESSING
The chemical composition of lunar samples returned by the Apollo missions has been determined, and a wide variation of percentages by weight of various constituents is apparent (refs. 38, 39).
Silica (SiO2) composes approximately 40-50 percent of the lunar soil, and in abundance are also found oxides of aluminum (Al203), iron (FeO), magnesium (MgO), calcium (CaO), and titanium (TiO2). Oxides of sodium (Na20), potassium (K20), phosphorus (P203), manganese (MnO), and chromium (Cr203) are present in less than 1 percent.
In the manufacture of window sheet and plate glass, soda-lime glass is most commonly used. Its composition includes approximately 71-73 percent silica (SiO2), 12-14 percent soda (Na20) and 10-12 percent lime (CaO) (ref. 40). Soda is absent from the lunar soil in percentages needed for producing commercial soda-lime glass and it proves to be a costly item to supply from Earth (4). Fortunately, it does not appear to be necessary to supply additional Na2O. Commonly used oxides in commercial glass which, if desired, would by necessity be additives to be mixed with the lunar materials, include lead oxide (PbO), used to provide X-ray and gamma-ray protection by absorption (ref. 41) and boric oxide (B20 3), used when good chemical resistance, high dielectric strength, and low thermal expansion are desirable (ref. 42).
A simplified diagram of industrial glass-making is given in figure 4-26 with lunar soil listed as the raw material to be provided.
Figure4-26.

After additives (if any) are mixed with the lunar soil, the acid leaching stage removes undesirable materials from the mixture, such as iron oxides which degrade the transmissivity of the glass. If it is decided to produce almost pure silica glass (> 95 percent SiO2) almost all of the non-silicate constituents are leached out with acid at this stage. The furnace temperature needed to melt pure silica (~ 1700 degrees C) is higher than that needed for soda-lime glass (~ 1550 degrees C) but is well within the limits of the solar furnace to be utilized. Requirements for providing these temperatures are calculated as follows:
Assumptions:
For silica glass, ~ 890 kW (640 m^2 solar collector) is needed. For soda-lime glass, ~ 870 kW (626 m^2 solar collector) is needed.
Volume requirements for the processing plant are governed basically by the volume needed for the melting tank and the annealing lehr. Although most industrial processes for plate glass involve capacities substantially greater than 40 t/day, the following estimates based upon a scaling down from larger systems are given as guidelines: approximately 23 m in length for the tank, 91-122 m in length for the annealing lehr, 1-2 m in depth, and, to allow for trimming, a width of 0.3-0.4 m in excess of the desired width of the glass panels. Note that some processes have annealing lehrs of only 10 m in length (ref. 44). For panels 0.5 m in width, the volume of the tank and lehr assembly using the upper values of the above dimensions is about 280 m^3. Additional space must be provided for the preliminary processing and cutting phases.
A rough estimate for the weight of a plant processing 40 t/day is 400 t.