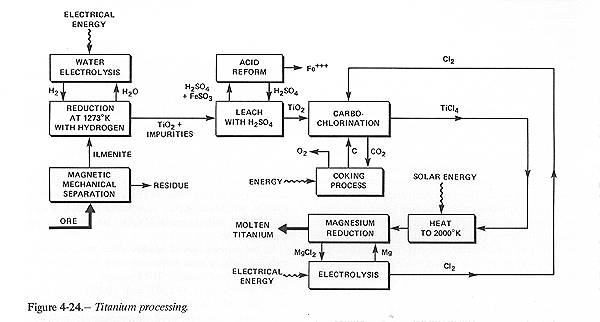
PROCESSING OF METALS Methods for Refining Titanium

Figure 4-24 demonstrates various means for obtaining titanium from lunar ore. It is reasonable to expect that ilmenite could be obtained from lunar ore since a similar process on terrestrial ore is carried out commercially using a combination of magnetic, electrostatic and flotation separators. This requires crushing, gravity and a flocculant which lead to complicated but not insurmountable problems common to any wet-chemistry process. A further complication may be the presence of magnetic glass formed during meteoroid impacts. At the next processing step the use of high temperature reduction of the ilmenite using hydrogen seems preferable.
The alternatives all require the consumption of carbon. On Earth this simply means the expenditure of coke, but in extraterrestrial processing it means that carbon must be recovered from carbon dioxide produced during the reduction or chlorination, which would have to be accomplished by high temperature reduction of the carbon dioxide with hydrogen. Obviously, it would save processing steps and mass if this process is applied directly to the ilmenite. The next processing step shown in figure 4-24 is the reduction of titanium dioxide. The appropriate method appears to be carbochlorination followed by reduction with magnesium to produce molten titanium. Important considerations are that magnesium is present in lunar ore and the production of titanium in liquid form makes continuous automated processing and alloying simpler to achieve.
Methods for Aluminum Extraction
The aluminum in lunar ore is in the form of plagioclase, (Ca,Na)(Al,Si)4 O8, while magnesium and iron remaining after ilmenite removal are in the form of pyroxene, (Ca,Fe,Mg)2 Si2 O6 These are not normal sources of aluminum, magnesium and iron on the Earth because of the difficulty of economically separating the desired materials from association with such a wide variety of other elements. Literature was surveyed, and researchers at Bureau of Mines consulted to discover by what means metals or their oxides could be extracted from low grade ores comparable to lunar soil. Only two processes were found. These can be used to obtain alumina from anorthosite.
Anorthosite is a rock composed of plagioclase feldspar with minor amounts of pyroxene and olivine and is similar to the material found on the lunar surface. Figure 4-25 shows these processes. The method of sodalime-sinter was eliminated because it consumes lime at a rate six times greater than it produces alumina, thereby requiring a disproportionate increase in plant size. The direct production of metals by electrolysis of molten anorthosite is often proposed, but the results of research have been discouraging. The remaining possibility is the melt-quench-leach process which in extensive laboratory tests has succeeded in recovering over 95 percent of the alumina present in the ore. In this process the ore is melted and then quenched to a glass. It is then treated with sulfuric acid to leach out the alumina component.
Further treatment of the aluminum sulfate follows standard procedures that have been developed for lowgrade bauxites and clays.
Figure 4.25

Figure 4-25 indicates that three paths are possible once alumina has been obtained. The Hall Process is unsuitable because it would be extremely difficult to automate, and it consumes its electrodes and electrolyte. The subchloride process is very attractive because of its simplicity. It consists of reacting aluminum chloride with alumina at high temperature to produce aluminum subohloride which later breaks down into aluminum and aluminum chloride. It has not been chosen as the baseline process because a pilot plant at Arvida, Quebec, was shut down when the process reportedly had difficulty on a large scale. The highly corrosive nature of the chloride vapor has been blamed for the failure 3. Of the two remaining processes, carbochlorination followed by reduction with manganese (Toth Process) is a good possibility. However, it is a batch process yielding a granular product which must be removed, melted, and cast, and requires an extra carbon reduction process. The high temperature electrolysis method is continuous and yields liquid aluminum ready for casting into ingots. For these reasons it has been chosen. However, research should take place into the possibility of a melt-quench process (no leach) followed by direct extraction with a subchloride process which, in theory, could reduce the plant mass by approximately 1/2.